1. Atom construction, constituents- their properties
2. Element (atom), ion, molecule
3. How electrons are distributed in atom shells?
4. Mass number, atomic number, and how they are written
together with the chemical symbol?
5. Solid, liquid, gaseous
6. What liquids conduct electricity and why?
7. Know a few chemical symbols
for some elements and their ions and their charges
8. Laboratory experiment
9. Questions
Atom construction, constituents -
their properties?
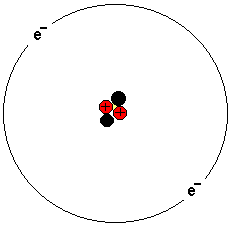
The picture shows a helium atom. It has two protons, two
neutrons and two electrons. The atom consists of a nucleus
and electrons orbiting around.
The atom nucleus consists of protons and neutrons. Protons
are positively charged and neutrons are neutrally charged.
The atomic weight is made up of essentially the atomic
nucleus. The task of the neutrons is to hold together the
protons.
Electrons are negatively charged. In an atom, the number of
electrons is exactly the same as the number of protons in
the nucleus.
If the nucleus would be as big as a “putter and ball” and
located in the middle of Ullevi Stadium (Sweden), then the
electrons would orbit the stands and would be the size of
pinheads. Most of the atom is thus void or vacuum.
An atom is so small that it can only be seen through an
electron microscopy. An electron microscope can magnify
500,000 times.
Element (atom) - ion -
molecule
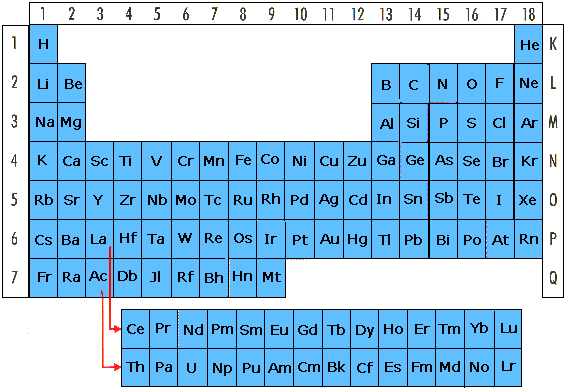
All elements are presented in the periodic system (table of
elements). Behind every element is only one kind of atoms.
The rows 1-7 are called periods. The atoms in each period
have an equal number of electron shells. In the first period
are hydrogen (H) and helium (He). Hydrogen has one electron
and helium has two electrons. They have both only one
electron shell. Their electron/s orbit the nucleus in the
same electron shell. Period 2 has 2 electron shells, period
3 has 3 electron shells etc...
The vertical columns 1-18 down are called groups. There are
18 groups. All elements in the same group have similar
properties.
Group 1 contains the alkali metals. They react violently
with water and giving away one electron, thus forming ions
having a charge of plus one (+1).
Group 2 contains the alkaline earth metals. They all want to
form ions with the ionic charge plus two (+2). This is
because they like to donate two electrons.
Group 17 contains the halogens. These like to react with the
alkali metals (group 1) and create salts. A halogen then
adds one electron from an alkali metal. The halogen gets the
charge minus one (-1) and the alkali metal gets the charge
plus one (+1). Example: a sodium atom donates one electron
to a chlorine atom. The sodium atom becomes an ion with the
charge plus one (+1). The chlorine atom that receives one
electron becomes also an ion, but with the charge minus one
(-1). The scenario can be demonstrated when one-piece of
sodium metal is placed in a container with chlorine gas. The
chlorine gas is lethal if inhaled. Similarly, the sodium
metal is dangerous to eat. When the substances react as
recently described sodium and chloride ions are created.
Together they form the salt sodium chloride that is used in
cooking. Sodium chloride is “common salt”. Common salt is
harmless when eaten in small quantities.
Group 18 far to the right contains the noble gases. They do
not want to react with other elements.
How electrons are distributed in atom shells?
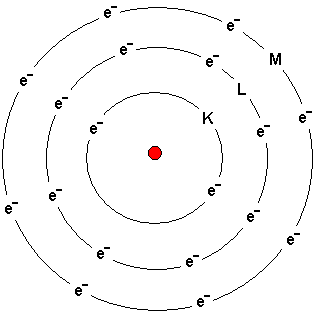
The atom above depicts Argon - a noble gas located on
the far right in the table of elements.
Electrons orbit the nucleus.
The shells are called from the nucleus outwards the K, L, M
and N shells. The names of the shells are shown in the
picture.
The K-shell contains a maximum of two electrons.
The L-shell contains a maximum of eight electrons.
The M-shell can initially contain a maximum of eight
electrons.
Electrons are added the different shells from inside and
outward. If the atom has six electrons, then two of them are
placed in the innermost K-shell and four are placed in the
L-shell.
An ion is an element that has in the nucleus more or fewer
electrons than protons. This makes an ion charged. There are
thus negative ions and positive ions. Positive and negative
ions tend to attract each other and form salts.
Molecules are atoms in gang. Examples of molecules are
carbon dioxide (CO2), nitrogen gas (N2) or ethanol (C2H5OH).
In its entirety, a molecule is not charged. The atoms in
molecules bind to each other because they share electrons. A
hydrogen molecule (H2) shares two electrons. One electron
comes from each hydrogen atom. All atoms have a certain
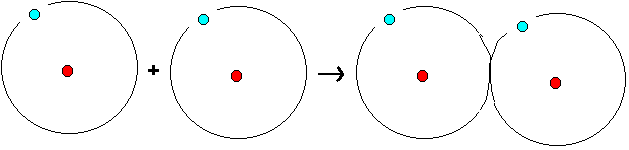
desire to form noble-gas-structure in their outermost shell.
The hydrogen atom wants to be similar to the helium atom.
Therefore, the hydrogen atom wants to let its outermost
shell (it has only one) have two electrons. This
noble-gas-structure is reached when every hydrogen atom
borrows one electron from one other hydrogen atom (see
below).
Look at the periodic system above. On the far right are the
noble gases. Helium at the top has two electrons in its
outermost shell. All noble gases below have eight electrons
in their outermost shell. Hydrogen atoms want to have two
electrons in their outermost shell, while most other atoms
in the periodic system desire eight electrons in their
outermost shell.
Mass number,
atomic number, and how they are written
together with the chemical symbol?
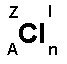
Above is the chemical symbol of chlorine (Cl). Around
is written a number of letters.
A = Atomic number. The atomic number indicates the number of
protons in the nucleus: 1H (hydrogen) and 2He (helium). The
atomic number increases in the periods above such as: 1H,
2He, 3Li, 4Be, 5B,
6C, 7N, 8O, 9F,
10Ne, 11Na, etc..
Z = Mass number. The mass number shows the number of protons
plus the number of neutrons in the nucleus. E.g. 1H
(hydrogen) and 4He (helium). Look in a more detailed
periodic table for the mass number.
l = Charge. If the element is in ionic form the charge is
shown here. Eg. Na+ and SO42-.
n = Number of atoms connected. Eg. H2 (hydrogen molecule),
F2 (fluorine molecule).
Solid, liquid and gaseous
A substance may be in solid, liquid or gaseous state. In
what state the substance is depends on temperature and what
substance it is. At “absolute zero” (0 Kelvin, 0 K) -273 °C,
the atoms in the substance do not move at all. At this
temperature all substances are in solid state.
Solid state:
As temperature increases the atoms start vibrating although
they are still in the same place and arranged in the same
structure. If we take water as an example, the atoms in the
ice have no movements at -273 oC. The temperature may be
increased even more and the atoms in the water molecules
start vibrating more and more. The water is still in solid
state.
Liquid state:
The temperature is now 0 oC, and the ice begins to melt. The
water turns to liquid state. In liquid state the molecules
in water have abandoned their subject ice structure and the
molecules are now moving freely in the water. All water
molecules are constantly getting new neighbors. The water
temperature rises to 100 oC. The water molecules are now
exchanging neighbors even more often since they have more
energy.
Gaseous state:
The temperature is now 100 oC. More energy is supplied the
water. The extra energy is absorbed by some water molecules,
which use it to free themselves from the solution. These
water molecules escape the liquid water. The water
temperature will not exceed 100 °C in the water. Water
molecules will absorb all extra energy added and escape the
solution. The water molecules turn into a gas. When all
water molecules have turned into gaseous state there is no
water left in the container. All water has evaporated.
What liquids conduct
electricity and why?
A solution conducts electricity if it contains ions.
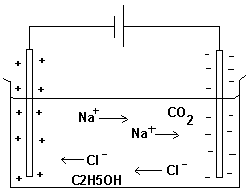
The tank above contains water with additions of sodium
chloride (NaCl), carbon dioxide (CO2) and ethanol (C2H5OH).
Charges of various kinds are attracted to each other. This
makes the negative terminal of the battery attract sodium
ions and the positive terminal attract chloride ions.
Ethanol molecules and carbon dioxide molecules that are not
charged will not move toward a charged terminal.
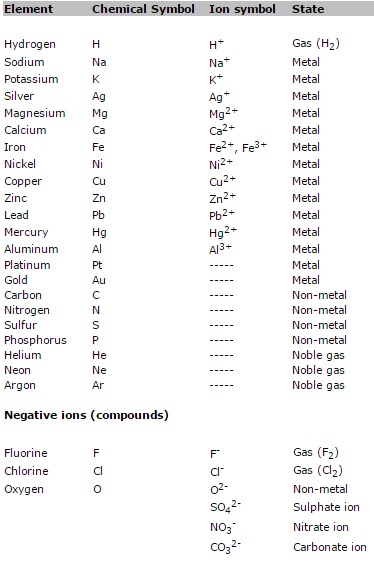
Know a few
chemical symbols for some
elements and their ions and their charges
Laboratory experiment
1.
Heat water in a crucible with a Bunsen burner – write a
laboratory report
2.
Heat potassium nitrate (KNO3) in the a crucible with a
Bunsen burner - write a laboratory report
3.
Heat sugar in a crucible with a Bunsen burner - write a
laboratory report
Discuss the results with the teacher
Questions
1.
How is an atom constructed?
2.
What constituents are there in an atom - which properties do
they have?
3.
What weighs the most in an atom? – protons or electrons?
4.
What is distinct for each period in the periodic system?
5.
What is distinct for each group in the periodic system?
6.
What is the difference between ion and atom?
7.
What is a molecule - explain?
8.
What is the difference between an atom and a molecule?
9.
What are halogens?
10.
What are noble gases - what are their properties?
11.
What properties do the alkali metals have?
12.
Why do some atoms join to form molecules?
13.
What is the atomic number?
14.
What is the mass number?
15.
What is solid, liquid and gaseous state?
16.
Tell what happens to water molecules when the temperature is
-273 degrees Celsius and slowly rises to 100 degrees
Celsius.
17.
Which liquids conduct electricity and why?
18.
Which ionic charge do usually the metal atoms have as ions?
19.
Why do the halogens always have the charge minus one as
ions?
20.
Why do the alkali metals always have the charge plus one as
ions?
21.
Explain how two lethal substances can react with each other
to form a harmless substance?
22.
Does ethanol conduct electricity - why?
23.
Does common salt (NaCl) conduct electricity - why?
Copywrite NGU, Northern Pontifical Academy 2025 (A.I.C.)