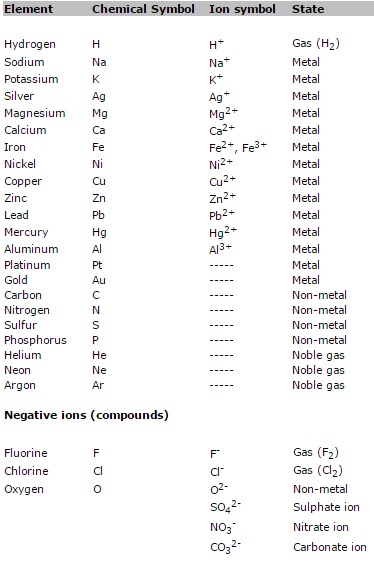
1. Chemical symbols; ion
charges of the most common ions
2. Electrochemical series
3. Oxidation and reduction
4. Redox reactions
5. Electrolysis
6. Electrode reactions and the PANC rule
7. Cation and anion
8. Electrolysis of various salt solutions. What happens
at the cathode and anode?
9. How is nickel plated or zinc plated?
10. Galvanic Elements (Batteries)
11. Effects explained by the electrochemical series
12. Questions
Chemical symbols of the
most common atoms; ion charges of the most common ions
![]()
Electrochemical series:
All metals in the periodic table are actually included in
the electrochemical series. In the electrochemical series
above are the metals that we are to work with. On the far
left are the base metals and the farther right you go, the
nobler the metals become. The metals to the left of hydrogen
(H) are called base metals, while the metals to the right
are called noble metals. If two different metals compete for
electrons, the noblest will win. All acids contain hydrogen
ions (H+) that dissolve base metals into ions. Noble metals
are not affected by acid attack.
Oxidation and reduction
Oxidation means that an atom or ion gives away one or more
electrons, thereby increasing the electrical charge, e.g.
oxidation of iron into iron ions.
![]()
Reduction means that an atom or ion adds one or more
electrons thus reducing the electrical charge, e.g.
reduction of copper ions into copper.
![]()
Redox reactions
When an oxidation takes place there is always a simultaneous
reduction. This is the basis of redox reactions. If we look
at the examples above, an oxidation can only occur when a
reduction occurs. If iron is immersed in copper ions (copper
sulphate) then both reactions occur simultaneously. The
reactions can be written together as follows.
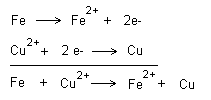
If you want to show electron transfer, this is done as
follows.
![]()
Copper is nobler than iron and copper therefore wins the
fight for the electrons
Electrolysis
With the help of electricity one can drive reactions. Below
is the electrolysis of hydrochloric acid (HCl). At the
negative terminal (cathode) hydrogen is created and at the
positive terminal (anode) chlorine gas is created. The
hydrogen ignites by the heat of a match (about 500 °C) and
the chlorine gas formed is toxic. The electrodes consist of
graphite.
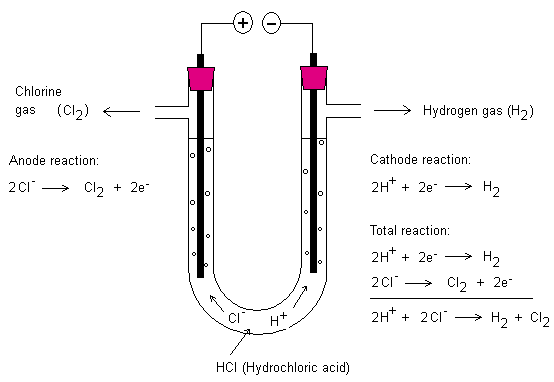
Electrode Reactions and the PANC-rule
Above are electrode reactions for the electrolysis of
hydrochloric acid. The positive terminal is an electrode and
the negative terminal is also an electrode. At these
electrodes chemical reactions occur. If the electrode is the
positive terminal it is called anode. The negative terminal
is called cathode.
To help you remember which electrode is which one can make
use of the PANC-rule. PANC stands for positive anode
negative cathode. The cathode is negative because the
battery above has electrons in the surplus that can be
delivered here.
The anode is positive since there is a deficit of electrons
here. Negative ions want to go to the anode. In the above
case the chloride ions (Cl-) want to go to the anode to
deliver one electron each. Chlorine gas is formed. Each
chlorine atom pairs up with another chlorine atom. This
allows the two chlorine atoms feel that they have eight
electrons each in their respective outermost shell. A
covalent bond is created and the molecule created is called
the chlorine molecule (Cl2).
Likewise at the cathode two hydrogen atoms pair up together
to form a hydrogen molecule (H2). This happens when each
hydrogen ion is added one electron.
Cation and anion
The ions wanting to the cathode (negative terminal) are
called cations, e.g. Na+, Mg+2, Ca+2, Cu+2, Zn+2, Al+3, i.e.
all positive ions.
Ions wanting to the anode (positive terminal) are called
anions, e.g. Cl-, F-, SO4-2, CO3-2, i.e. all negative ions.
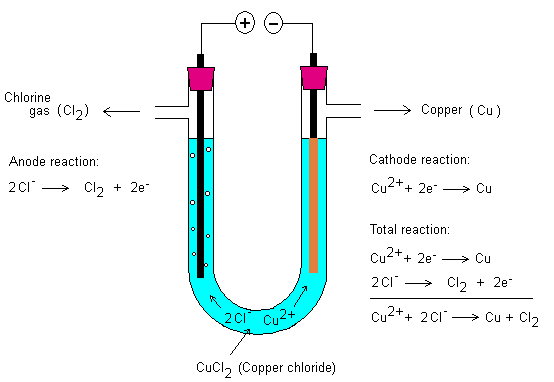
Electrolysis of various
salt solutions ... what happens?
Know theoretically what happens when different solutions are
electrolyzed. What will happen to the cathode and anode?
Below is electrolysis of copper chloride.
How is nickel plated or zinc plated?
This can be done by means of electrolysis. Use zinc ions
(Zn2+) or nickel ions (Ni2+) in solution. Let the metal
object that is to be zinc plated or nickel plated act as
negative terminal (cathode). Use the DC and start the
process. Electrons will then be located here and reduce zinc
or nickel ions to zinc or nickel.
Galvanic Elements (Batteries)
All the metals in the electrochemical series have different
abilities to oxidize other metals, i.e. get the other metal
to give away electrons that they want themselves. A metal
that oxidizes another metal is known as an oxidizing agent
and the metal that loses the electron or electrons is then
oxidized.
Similarly, a metal can be a reducing agent if it instead
gives away electrons to another metal. The other metal is
reduced, i.e. has got a lower charge.
E.g.
![]()
Copper ions: oxidizing agent (oxidizes iron atoms). The
copper ions are reduced to copper.
Iron: reducing agent (reduces copper ions). The iron atoms
are themselves oxidized to iron ions.
You can let the two metals compete in the “boxing ring” and
fight for the electrons. The noblest metal will win the
electrons and the weaker will lose theirs. The different
ability of the metals to add electrons is measured in
comparison to hydrogen, which constitutes the border between
noble and base metals. The capacity is measured in volts
(voltage). The two metals are compared on the capacity and
there will be an electron current in the conduit between the
electrodes.
Both metal pieces in the liquid below are made of two
different metals. The electrode giving electrons to the
other metal trough the conduit is the negative terminal
(cathode). The electrode receiving electrons is the positive
terminal (anode). The voltmeter will show a voltage. We have
created a battery. If one of the electrodes would have been
copper (anode) and the other would have been iron (cathode),
the voltmeter would have shown approximately 0.3 to 0.4 V.
If one had been a silver electrode (anode) and the other a
zinc electrode (cathode), the voltmeter would have shown
approximately 1.50 to 1.60 V. The ion concentration between
the electrodes also affects to some extent what voltage
there will be between the electrodes. From table collections
one can calculate the voltage between electrodes of
different metal types. Here, the ion concentration in the
electrolyte is often 1 mol/dm3 (1 molar) and the atmospheric
pressure is 1 bar.
Effects explained by the electrochemical
series
If you are to nail a copper roof, you should use copper
nails. If you use iron nails, then the iron will donate
electrons to the copper as described above. There is always
some moisture with salts between the copper and the nails of
the roof. A galvanic current will be created, and iron nails
will oxidize into iron ions.
Questions:
1.
Write the chemical symbol of the sulphate ion, nitrate ion
and carbonate ion?
2.
What does the electrochemical series show?
3.
Why can we arrange all the metals in the electrochemical
series - what capacity is compared?
4.
What metals are base?
5.
What metals are noble?
6.
What is the border between noble and base metals?
7.
What is oxidation?
8.
What is reduction?
9.
What is a redox reaction - give also an example of a redox
reaction. Show also the electron transfer.
10.
Describe the electrolysis of hydrochloric acid? Draw the
layout and write down the electrode reactions and the total
reaction.
11.
How could you test what gases are formed at the two
terminals in the question above?
12.
Describe the electrolysis of copper chloride? Draw the
layout and write down the electrode reactions and the total
reaction.
13.
How could you see what substances are formed at the two
terminals in the question above?
14.
What is a galvanic element?
15.
What do both metals compete about in a galvanic battery?
16.
How do you measure that?
17.
Which electrode (anode or cathode) will always win
electrons?
18.
Which electrode (anode or cathode) will always lose
electrons?
19.
Why should you nail a copper roof with copper nails?
20.
Why should you not nail an iron roof with copper nails?
21.
How do you cover a metal-object with zinc?
Copywrite NGU, Northern Pontifical Academy 2025 (A.I.C.)