1. Carbon occurs naturally with three forms in nature
...Diamond
...Graphite
...Fullerene
2. What is an organic compound?
3. Which is the hardest form of carbon and its softest -
what is the difference?
4. Alkanes (methane series) until octane
5. Alkenes until octane
6. Alkynes until octyne
7. Alcohols until octanol
8. Organic acids until octanoic acid
9. Know how a covalent bond works
10. Questions
Carbon occurs naturally with three
forms in nature
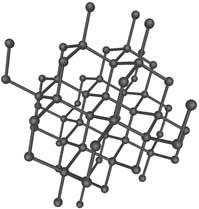
1. Diamond. (Info about picture). Only carbon builds up this crystal. Each carbon
atom binds to four other carbon atoms in a completely
regular three-dimensional structure. Diamond is the hardest
of all known minerals.
Application: jewelry, as abrasives in drill cores.
Formation: Formed under high pressure and temperature in the
Earth's mantle quite deep into the earth.

2. Graphite. (Info about picture). Each carbon atom
binds to three other carbon atoms in a hexagonal pattern.
Each corner consists of a carbon atom. This pattern is
spread as sheets and these sheets form layers. Weak bonds
keep these sheets together. This means that graphite is one
of the softer minerals on earth.
Application: in pencils - the blacking is scraped off as
layers that stick to the paper. Graphite conducts
electricity poorly and is therefore used in electrical
circuits as resistors. Graphite can burn and thus be used as
fuel.
Formation: Graphite forms when plant parts and dead animals
decompose in the absence of oxygen. This often happens while
the material is packed together under sediment loads of
eroded rock.
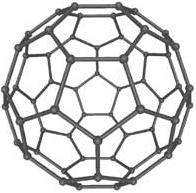
3. Fullerene. (Info about picture). Each carbon atom
binds to three other carbon atoms in patterns of hexagonal
and pentagonal rings. In each corner is a carbon atom. These
hexagonal and pentagonal structures build up forms that look
like footballs and small tubes.
Application: Scientists are investigating the possibility to
produce screens made of nanotubes. The screen is expected to
be thinner than a sheet of paper.
What is an organic compound?
An organic compound is a chemical compound containing the
element carbon, e.g. methane, chlorophyll or B-vitamin.
Which is the hardest form of carbon and its softest - what
is the difference?
The "hardest" form of carbon is diamond. It is very durable
because each carbon atom binds to four other carbon atoms in
a three-dimensional symmetrical network.
The softest form of carbon is graphite. It is soft since the
carbon atoms are positioned in layers that can easily
blacken off. Weak bonds hold together the layers.
Alkanes (methane series) until octane
All lines between the atoms below are symbols of covalent
bonds. A covalent bond includes two electrons. Below are
displayed structural formulas for alkanes.
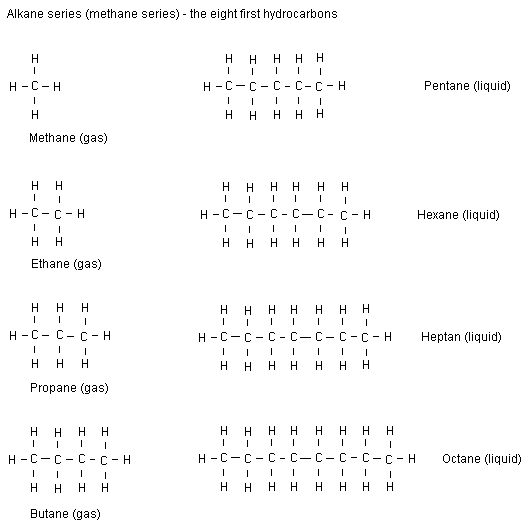
Of course the series continues and even more carbon atoms
can be connected in series. All compounds included in the
methane series are called alkanes. Note that all alkanes end
with ane. The more carbon atoms there are in a row, the
heavier they are. The breakpoint between the gaseous and
liquid alkanes at room temperature (25 °C) is between butane
and pentane. Alkanes with 17 carbon atoms or more are solid
in room temperature.
Alkenes until octene
Alkenes are alkanes that have received a double bond. All
alkene names end with ene. Below are three examples:
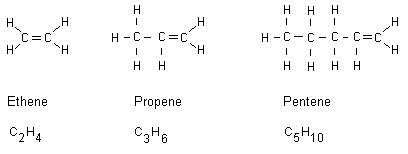
Of course, the double bond instead can exist between two
other of the carbon atoms in the chain. Note that a carbon
atom only can bind with four covalent bonds. A double bond
keeps more energy than a conventional single bond. When
double bond compounds burn they release more energy compared
to hydrocarbons having only single bonds. The above alkenes
burn releasing higher temperatures compared to alkanes.
Alkynes until octyne
Alkynes are alkanes having received a triple bond. All
alkyne names end with yne. Below are three examples:
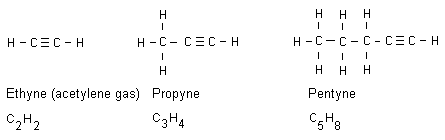
A triple bond keeps even more energy compared to a double
bond. Ethyne gas (acetylene gas) burns with a temperature of
more than 3000 oC. This makes the gas appropriate to use
when welding, when it is needed a high temperature.
Acetylene gas is stored in gas tubes. The gas is released
with a controlled flow rate and the gas can thus be ignited.
The flame is blue, intense and extremely hot.
Alcohols until octanol
An alcohol is an alkane that has replaced a bonded hydrogen
atom with an OH group. All alcohol names end with ol. These
compounds are then called alcohols.
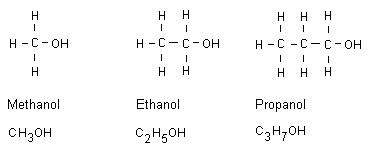
Methanol
Methanol is very toxic. Even when having drunk a small
amount of fluid one becomes blind, and at a slightly higher
dose one dies. If one has been exposed to methanol poisoning
one should drink ethanol and also larger doses of
bicarbonate. Detoxification occurs at the doctor. Methanol
has at times been confused with ethanol, which is part of
beer, wine and liquor.
Ethanol
Available as an intoxicant in beer, wine and liquor. Ethanol
is a poison.
Organic acids until octanoic acid
A carboxylic acid is an alkane that has replaced a bound
hydrogen atom and forming a COOH group. All acids with this
COOH group are called carboxylic acids.
Methanoic acid (formic acid)
Methanoic acid is revealed when terrorizing ants in an
anthill. If thereafter closing in with your nose, you can
feel a pungent odor. If you have a wound on your hand and
holding it close to the anthill, you can also feel the wound
stinging. An ant bite also reveals in the bite the sting of
formic acid.
Formic acid is used as a preservative for silage. Silage is
grass and hay stored in large silos on farms. In order for
the beneficial lactic acid bacteria to take over in these
silos, it is added a certain amount of formic acid. These
silos can then store grass and hay for the winter and the
cows and horses can have good food even then.
Ethanoic acid (acetic acid)
Ethanoic acid can be used as a preservative for cucumber and
as part in window cleaner.
Know how a covalent bond works
A covalent bond is also called electron-pair bond. A
covalent bond is a way for e.g. a carbon atom to lend an
electron to another atom, and a way for the other atom to
share its electron. This way both atoms experience they have
an extra electron. Covalent bonds are used so that the
joining atoms may have their outermost shell resemble the
outermost shell of the noble gases, i.e. the atoms get noble
gas structure. Hydrogen then has two electrons in its
outermost shell resembling the helium atom (He). The carbon
atom then has eight electrons in its outermost shell
resembling the other noble gases. The noble gases are placed
to the far right in the periodic system.
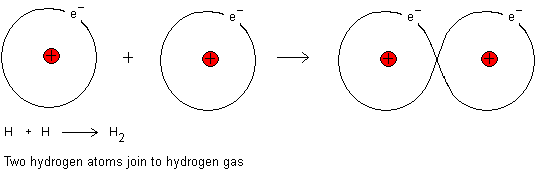
Both hydrogen atoms experience they have two electrons. If
you follow from hydrogen in the periodic table and further
to the right, you can see that the last atom in the period
is helium. Helium has two electrons in its outermost shell.
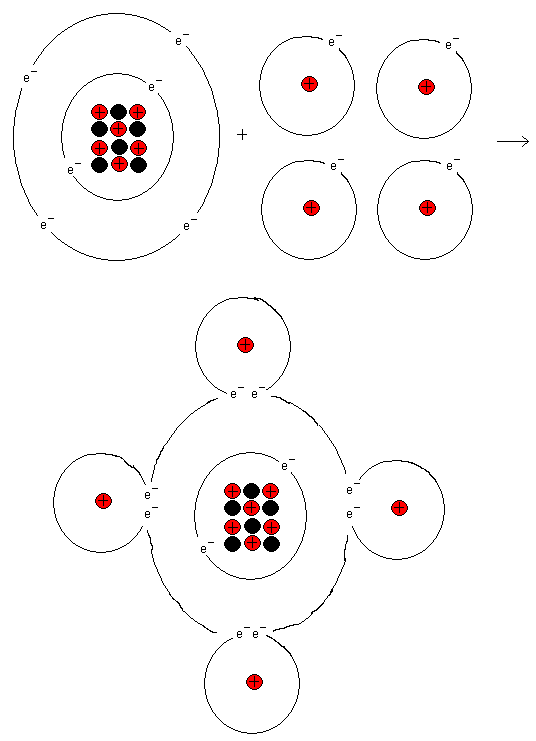
The outermost shell of a carbon atom wants to have eight
electrons. From the start, it has four of them, but if the
carbon atom binds to four hydrogen atoms, it will have eight
electrons in its outermost shell. The carbon atom then gets
noble gas structure and resembles Neon. Follow in the
periodic system from carbon and out to the right. Here you
find the element Neon (Ne).
Similarly, the hydrogen atoms also get noble gas structure.
They experience they have two electrons each.
The atoms become more stable when they have an outer shell
that looks like a noble gas outermost shell. Hydrogen gas is
so stable that it can be found in the atmosphere, although
much of the hydrogen gas content in our atmosphere has
disappeared into space, since the gas is so light.
Also methane gas is stable and can be found in our
atmosphere. Methane is 25 times more aggressive as a
greenhouse gas compared to carbon dioxide that is more
associated as a greenhouse gas. Greenhouse gases contribute
to global warming.
Questions
1.
Draw the structural formula of methane, ethane, and hexane?
2.
Where is the boundary between the gaseous and liquid
hydrocarbons?
3.
Where is the boundary between liquid and solid hydrocarbons?
4.
Write the chemical symbol for ethane and octane?
5.
Give a joint property of all alkenes?
6.
Give a property that is the same for all alkenes?
7.
Give a joint property of all alkynes?
8.
Give a property common for all hydrocarbons having a double
or triple bond?
9.
Write the chemical symbols for ethyne and propene?
10.
What is required by a molecule to be called alcohol?
11.
One of the alcohols is sometimes strangely mixed up with
ethanol - which one?
12.
What should one do if a person has been poisoned by
methanol?
13.
Write the chemical name for propanol and hexanol?
14.
What is the same for all carboxylic acids?
15.
Draw the structural formula for octanoic acid and propanoic
acid?
16.
What is a covalent bond?
17.
Show how a covalent bond works in an ethylene molecule?
18.
What happens if you mix an organic acid with an alcohol?
19.
Why does it not remain so much hydrogen in the atmosphere
although the hydrogen molecule is quite stable?
Copywrite NGU, Northern Pontifical Academy 2025 (A.I.C.)